
Amy Parr | News Editor
December 6, 2020
Rumors of a Covid-19 vaccine have been running rampant for months, but in recent weeks there has been more concrete news regarding the timeline of the distribution of the new vaccine.
Formulating a vaccine in such a short period of time is historically unprecedented, but new scientific techniques are aiding in the rapid development of vaccines. For example, Moderna, one of the companies at the front line in the race to develop a vaccine, used RNA technology, which commands cells to generate certain proteins through genetic instruction. As a result of this new approach, Moderna’s vaccine has a 95% success rate at preventing infection with Covid-19. The FDA is going to review Moderna’s data on December 17 in order to determine whether or not to grant emergency approval.

Another front-runner in this vaccination race is BioNTech, a German company that partnered with Pfizer at the beginning of the pandemic. Their vaccine also used RNA messenger technology, resulting in a similar 95% success rate in the last trial of the vaccine.
The U.K. authorized BioNTech’s drug on December 2, and 20 million vaccines are expected to be administered in Britain soon. President Donald Trump hinted that this vaccine would be approved in the United States prior to the election, hoping to win some last-minute votes, but unfortunately the FDA isn’t scheduled to evaluate this vaccine until December 10, more than a month after election day.

“I think that there’s a good chance that the vaccine will be approved by the FDA since the success rate was so high in the trials,” junior Evan Lipofsky said. With both vaccines at a 95% success rate and already having been approved in the U.K., chances are that the FDA will follow suit. (yugatech.com)
Despite the fact that the FDA hasn’t made a decision, the United States has already ordered 100 million doses, spending $1.95 billion.
We won’t reach herd immunity for months, which is why the distribution of the vaccine once it reaches approval will be critical in curbing the spread of the virus. The first group to be vaccinated will be the valuable healthcare workers and those working in nursing homes, which is estimated to be sometime later this month. The next group of people to receive the vaccine are essential workers, including teachers and those working in transportation, as well as those that are more susceptible to the virus (the elderly and people with pre-existing conditions).
“Since my family is high risk, it’s comforting knowing that we’ll be getting the vaccine sooner rather than later since we’ve been so anxious about catching it for the last few months,” junior Madeline McDonald said. 2020 has been rough for everyone, but especially for those who have had to worry about the more serious repercussions of catching the virus.
This second wave of vaccinations is expected in February and March of next year. If all goes according to plan, the vaccine won’t be available to the general public until spring 2021.
Of course, there are going to be people who refuse to be vaccinated because personal beliefs against medical science, which is often founded on misinformation and could potentially cause a major problem.
“Just because the vaccine was produced faster than normal doesn’t make it any less safe,” Lipofsky said, “so people shouldn’t be afraid of taking it.” The FDA wouldn’t approve of a vaccine unless they knew it wouldn’t be harmful, which is why fears surrounding the vaccine are baseless and a result of paranoia more than actual scientific concern.
There’s a possibility that if we reach herd immunity, it won’t matter that certain groups refuse the vaccine. Herd immunity greatly diminishes the risk of transmission.
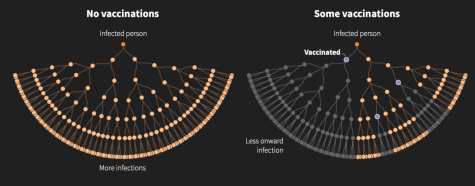
Unfortunately, there is no clear answer as to when herd immunity can be reached with Covid-19 because the percentage of those immune to the disease in order to reach herd immunity is different compared to past viruses. For example, 95% of the population needs to be immune for the measles, while polio requires just 80%. Either way, a strong majority needs to be immune in order for life to return to normal.
“I just hope that whenever a vaccine gets approved, life can go back to normal,” McDonald said. “We could all use a little good news right now, and I think that the promise of a vaccine will boost everyone’s spirits.”

I’ve had a lot of discussions in my community about the way the vaccine will function – Thank you for providing some description on how it works to the student body! I think most people aren’t knowledgeable of what mRNA is and how the vaccine will work, so providing a base understanding is critical to addressing the fear surrounding the vaccine.